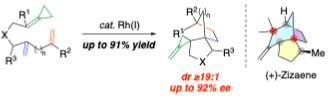
PhD student, Yu Zhu, presents Stereoselective Intramolecular Rhodium-Catalyzed [(3+2+2)] Carbocyclization Reactions of Alkylidenecyclopropanes (ACPs) for the Concise Synthesis of Bridged Tricyclic Products
Date
Friday February 21, 202511:30 am - 12:00 pm
Location
Chernoff Hall, Room 117Stereoselective Intramolecular Rhodium-Catalyzed [(3+2+2)] Carbocyclization Reactions of Alkylidenecyclopropanes (ACPs) for the Concise Synthesis of Bridged Tricyclic Products
ABSTRACT:
Transition metal-catalyzed higher-order carbocyclization reactions with alkylidenecyclopropanes (ACPs) provide a powerful strategy for the chemo-, regio-, and stereoselective construction of complex polycyclic scaffolds. While the diastereoselective synthesis of fused bicyclic and tricyclic systems has been explored, the stereoselective assembly of the more challenging bridged tricyclic skeletons–found in numerous bioactive sesquiterpenes–remains underdeveloped. This seminar will present the development of a diastereo- and enantioselective intramolecular rhodium-catalyzed [(3+2+2)] carbocyclization reaction involving ACPs tethered to skipped dienes, enabling the formation of bridged tricyclic products with up to three new quaternary stereocenters in a single step. The first part will examine the diastereoselective reaction, showcased in a concise stereoselective total synthesis of the sesquiterpene (+)-zizaene. The second part will describe the development of a more demanding enantioselective variant, leveraging a novel asymmetric chiral counteranion-directed catalysis (ACDC) strategy. This approach enables asymmetric induction through the outer-sphere chiral anion of the catalyst, providing an alternative to conventional inner-sphere chiral ligand control. Overall, this work highlights the potential of the ACDC strategy to facilitate other enantioselective metal-catalyzed higher-order [m+n+o] carbocyclization reactions of ACPs, expanding the toolbox for the synthesis of complex polycyclic systems.