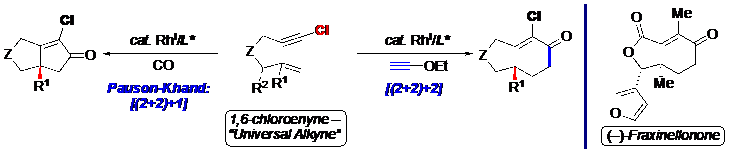PhD student, Michael Ylagan presents Stereoselective Rhodium(I)-Catalyzed Carbocyclization Reactions with 1,6-Chloroenynes – The “Universal Alkyne”
Date
Friday November 3, 202311:30 am - 12:30 pm
Location
Chernoff Hall, Room 117Ph.D. Seminar Title:
Stereoselective Rhodium(I)-Catalyzed Carbocyclization Reactions with 1,6-Chloroenynes – The “Universal Alkyne”
Stereoselective transition metal-catalyzed carbocyclization with 1,6-enynes provides an attractive approach to constructing elaborate cyclic scaffolds in several important natural products. Currently, most examples are limited to 1,6-enynes with terminal olefins, which can be ascribed to the poor reactivity and selectivity with higher olefin homologs, e.g., 1,1-disubstituted alkenes. The seminar will outline the development of two enantioselective transition metal-catalyzed carbocyclization reactions, namely the [(2+2)+1] and [(2+2)+2] reactions with 1,6-chloroenynes with challenging 1,1-disubstituted alkenes to afford enantioenriched bicyclopentenones and bicyclohexenones respectively. We will provide insight into the role of the alkynyl chloride in addressing this long-standing problem and showcase the synthetic utility with a concise and stereoselective total synthesis of the limonoid (–)-fraxinellonone. Hence, we intend to provide compelling evidence for a “Universal Alkyne” that facilitates challenging carbocyclization reactions.