PhD Student, Mai-Jan Tom presents Regioselective and Stereospecific Rhodium-Catalyzed Allylic Cyanomethylation: Construction of Acyclic b-Quaternary Stereogenic Nitriles
Date
Friday September 23, 202211:30 am - 12:00 pm
Location
Chernoff Hall, Room 117Regioselective and Stereospecific Rhodium-Catalyzed Allylic Cyanomethylation: Construction of Acyclic b-Quaternary Stereogenic Nitriles
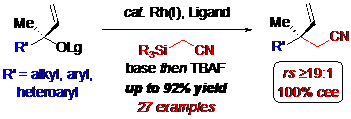
Alkyl nitriles are important motifs frequently embedded in an array of bioactive natural products and pharmaceuticals, in addition to being versatile synthetic intermediates. Nevertheless, methods for their selective incorporation are underdeveloped compared to related motifs, namely, aldehydes, ketones, esters, amides, etc. Consequently, the ability to readily employ alkyl nitriles as pronucleophiles in asymmetric transition metal catalysis is highly desirable, albeit challenging because of the nature and reactivity of the anion. This seminar will describe the development of a highly regioselective and stereospecific rhodium-catalyzed cyanomethylation of tertiary allylic carbonates for constructing acyclic b-quaternary stereogenic nitriles, which permits access to several carbonyl derivatives that are challenging to prepare using conventional pronucleophiles. The synthetic utility of the stereospecific cyanomethylation is further highlighted through derivations of the nitrile group, one of which provides a formal synthesis of two natural products.