PhD Student, Derek Esau presents Controlled-Atmosphere Flame Fusion Growth and Preparation of Non-noble Transition Metal Crystal Electrodes for Electrochemical and Electrocatalysis Research
Date
Friday October 14, 202211:30 am - 12:30 pm
Location
Chernoff Hall, Room 117Controlled-Atmosphere Flame Fusion Growth and Preparation of Non-noble Transition Metal Crystal Electrodes for Electrochemical and Electrocatalysis Research
Derek Esau (Mr./He/Him)
Dr. Gregory Jerkiewicz Laboratory
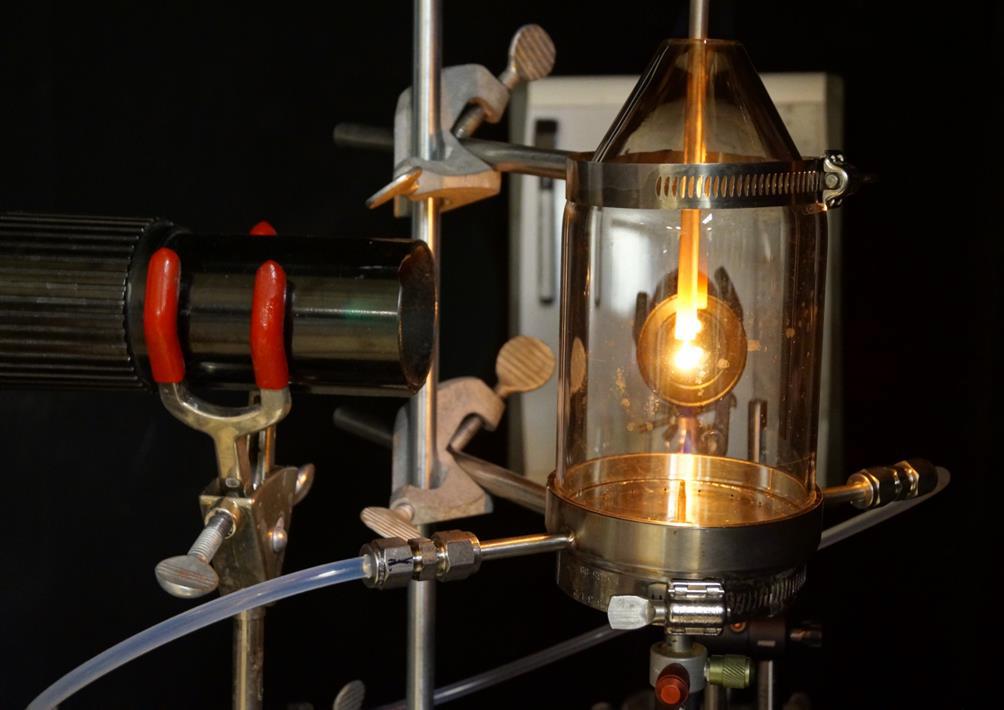
Flame fusion (melting) methodologies for growing high quality single crystals of noble metals (namely Au(hkl) and Pt(hkl)) has been a hallmark of interfacial electrochemistry and electrocatalysis for the past four decades, allowing researchers to investigate surface specific phenomena. However, attempts in growing non-noble metal single crystals using flames in air resulted in rough, highly oxidized and unstable melts, that eject material from the surface causing sparking. A growth chamber was designed to allow for an inert gas to be released into an open-top, quartz cone. This design allows for hot gases to be expelled from the chamber while maintaining a slightly reducing atmosphere where oxygen sensitive metals can be stably melted. The design was successfully implemented and has most notably allowed for the growth of Ni, Cu, Co and Fe poly-oriented single crystals (POSCs).Wulff construction calculations were preformed using density functional theory to model the equilibrium shape of the newly grown metallic crystals. Scanning electron microscopy was used to characterize the surface of the POSCs where unique growth and faceting behaviour were observed. The crystallinity of the POSCs has been verified using Laue X-Ray backscattering. Cutting, polishing and surface pre-treatment using induction annealing and a state-of-the-art water droplet transfer system has allowed for oxide-free, electrochemical measurements to be conducted on metallic Ni POSSCs and the basal low Miller index surfaces namely, Ni(111), Ni(110) and Ni(100). The cyclic voltammetry profiles in 0.10 M aqueous NaOH of the aforementioned surfaces reveal interesting features associated with their unique surface structure, thus validating the methodology for electrochemical and electrocatalysis research.