Dr. Dennis G. Hall presents From Drugs to Catalysts: Design and Application of Cyclic Hemiboronic Acids Guided by Properties and Reactivity
Date
Friday August 18, 202311:30 am - 12:30 pm
Location
Chernoff Hall, Room 117From Drugs to Catalysts: Design and Application of Cyclic Hemiboronic Acids Guided by Properties and Reactivity
Dennis G. Hall
Department of Chemistry, University of Alberta,
Edmonton, AB, Canada, T6G 2G2
dennis.hall@ualberta.ca
ABSTRACT
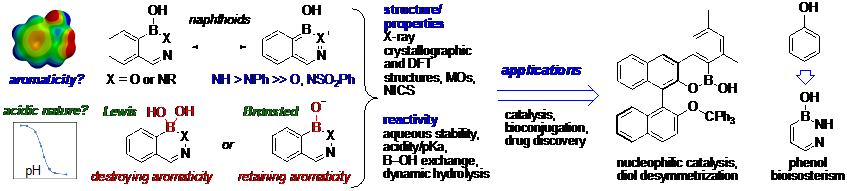
The commercialization of benzoxaborole drugs has sparked a renaissance surrounding heterocycles derived from boronic acids in organic and medicinal chemistry, where they demonstrate a wide range of biological properties. Despite this success, many questions remain unanswered regarding the physical properties, acidic nature (Lewis vs Brønsted), dynamic behavior, and reactivity of boranol (B–OH)-containing heterocycles in organic and aqueous media. To resolve decades of conflicting views on the acidic and aromatic characteristics of pseudoaromatic hemiboronic acids, our laboratory reported multipronged experimental and computational approaches.1-2 These fundamental studies are essential for guiding a systematic application of select boroheterocycles as enantioselective reaction catalysts,3 in bioconjugation, and as new antibacterial drug chemotypes and bioisosteres of pharmaceutically important classes of heterocycles. In recent work, benzoxazaborine derivatives were identified as modular scaffolds enabling both nucleophilic and electrophilic catalytic activation of alcohols.4 Taking from the ‘naphthoid’ benzodiazaborines, studies on the simpler ‘benzenoid’ diazaborines highlights their potential role as phenolic isosteres. Ongoing work investigates the exchangeability of B–OH bonds for direct catalytic activation of alcohols and diols under thermal and photochemical conditions. Altogether, these studies lay the foundations that will guide the future applications of cyclic hemiboronic acids in catalysis, bioisosterism, and medicinal chemistry.
REFERENCES
[1] Kazmi, M. Z. H.; Rygus, J. P. G.; Ang, H. T.; Paladino, M.; Johnson, M. A.; Ferguson, M.; Hall, D. G. J. Am. Chem. Soc. 2021, 143, 10143–10156.
[2] Ang, H. T.; Ponich, A. A.; Paladino, M.; Miskolzie, M.; Hall, D. G. J. Am. Chem. Soc. 2022, 144, 10570–10581.
[3] Estrada, C. D.; Ang, H. T.; Vetter, K.-M.; Ponich, A. A.; Hall, D. G. J. Am. Chem. Soc. 2021, 143, 4162–4167.
[4] Rygus, J. P. G., Hall, D. G. Nat. Commun. 2023, 14, article 2563.